Kwon Lab receives patent for a promising new therapy with implications for many fibrotic conditions
By Nicole Etter
Kidney disease. Heart failure. Diabetes. COVID-19. In all these conditions and more, the body’s normal healing process can run amok, leading to fibrosis, an excessive accumulation of connective tissue in inflamed organs, including the liver, lungs, pancreas, heart and other parts of the body. In the U.S., an estimated 45 percent of deaths are due to fibrosis.
But a promising potential therapy developed in collaboration with University of Wisconsin–Madison School of Pharmacy could halt fibrosis, improving outcomes for a variety of inflammatory diseases. The U.S. Patent Office recently awarded the Wisconsin Alumni Research Foundation (WARF) a patent for the work — the culmination of years of collaboration between Professor Glen Kwon, the Jens T. Carstensen Distinguished Chair in Pharmaceutical Sciences; senior scientist Bianca Tomasini-Johansson; and Pawel Zbyszynski (PhD ’19), a former member of the Kwon Lab, along with their collaborators in the UW School of Medicine and Public Health.
“To me, its development path is a reminder of the vibrant research environment present on the UW–Madison campus and the continually present Wisconsin Idea.”
— Pawel Zbyszynski
“To me, its development path is a reminder of the vibrant research environment present on the UW–Madison campus and the continually present Wisconsin Idea,” says Zbyszynski, who relocated to Missouri after graduating from the School of Pharmacy to work as a senior formulations scientist for Mallinckrodt Pharmaceuticals.
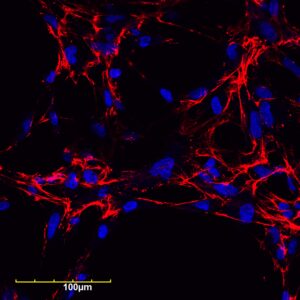
The research team focused on fibronectin fiber deposition as a novel drug target. An increase in fibronectin and collagen in chronically inflamed tissues can cause a buildup of scar tissue, which can then trigger other problems.

“Fibronectin is a protein that is in blood, connective tissue, and most cells, but it’s increased when there is some inflammation or fibrosis,” Tomasini-Johansson explains. “The fibronectin and collagen-containing extracellular matrix becomes dense and not pliable, and then cells that are supposed to be working start dying because the tissue they’re sitting on has become too dense and hard.”
The team developed a PEGylated functional upstream domain peptide (PEG-FUD), which tightly binds to fibronectin and inhibits the development of the fine fibrils that precede collagen deposits, preventing the formation of scar tissue. Through daily dosing by subcutaneous injection, PEG-FUD is absorbed into the body’s circulation.
“Continued research on PEG-FUD will provide important insights into the role of extracellular fibronectin in fibrosis and cancer,” Kwon notes.
While still in pre-clinical studies, PEG-FUD paves the way for more effective treatments for fibrotic diseases. “Our hope is that this would be something that really stops the fibrosis so you don’t have to keep taking it for the rest of your life,” Tomasini-Johansson says.
Decades in the making
The work builds on years of study by Deane Mosher, a professor of biomolecular chemistry in the UW School of Medicine and Public Health. Tomasini-Johansson first picked up the work when she returned to UW–Madison as a staff scientist a few years after graduation. She began working with bacterial peptides to determine whether they could affect the assembly of fibronectin, a key step to understanding how to keep normal tissue from turning fibrotic.
“One of these peptides in particular could decrease the assembly of fibronectin quite strikingly. Very small amounts were effective,” explains Tomasini-Johansson, who first published on the topic in 2001. “We kept using the FUD peptide for understanding different areas of fibronectin assembly.”
The early work focused on understanding the mechanisms of fibronectin assembly, but Tomasini-Johansson was intrigued by FUD’s potential clinical use as well. She moved to the lab of Hans Sollinger in the UW Department of Surgery and then began testing FUD in a kidney fibrosis model. But she ran into a wall.
“By itself, FUD wasn’t working very well,” she says. “I became concerned it wasn’t reaching the kidney as well as it should, so I started looking for ways to protect the peptide so it could reach the kidney better. And that’s how I found Dr. Kwon.”
Improving drug delivery
Kwon is an expert in using polymeric nanotechnology to improve drug delivery, and Kwon, Tomasini-Johansson, and Zbyszynski, then a Pharmaceutical Sciences graduate student in Kwon’s lab, began collaborating in 2014 to see if they could improve the FUD peptide. Working under the mentorship of Kwon, Zbyszynski attached polyethylene glycol (PEG) polymers to FUD, a process known as PEGylation.

“PEGylation is one method that can be used to improve the way in which the drug works,” Zbyszynski explains. “PEGylation increases the molecular weight and effective radius of the drug. We increased the size and molecular weight of FUD by quite a significant margin, intending to make it stay in the blood longer. If it stays in the blood longer, it could be more effective.”
Zbyszynski tried different variants until he found one that successfully increased the longevity of the drug, which they then tested in a kidney disease model. The team was thrilled at how well the PEGylated form worked, and they published their findings in 2018.
“It was very, very effective. We could decrease fibronectin, collagen, and some immune cells in the tissue,” Tomasini-Johansson says. “It is surprising how resilient this peptide is and how effective it is.”
Working toward clinical trials
In November 2020, the U.S. Patent Office awarded a patent for the team’s “composition and methods for the inhibition of fibrosis.” Zbyszynski was honored to be listed as an inventor on a patent for work he produced before graduation.
“It’s a very humbling experience,” he says. “Being a graduate student has its challenges, and it’s just so incredibly rewarding to have the fruits of your labor not only produce good results, but also be successful and consequential. And it was a cherry on the cake to get validation from the U.S. Patent Office as well.”
And the work continues. There’s still much more to learn about fibronectin inhibition as a strategy for treating fibrosis, cancer, wound remodeling disorders, and more, Zbyszynski notes.
“We believe there’s a lot of potential to explore,” says Zbyszynski, who is no longer involved in the hands-on work but who stays in touch about the research with his former collaborators.
Other collaborators on campus include Associate Professor Nathan Sandbo from the UW Department of Medicine, who is testing PEG-FUD in a pulmonary fibrosis model.
“I think one of the things that’s very unique about UW–Madison is you are able to have interactions with people of different backgrounds,” says Zbyszynski. “You get to do interdisciplinary research that allows the convergence of ideas that in other environments could live in their own silos. It allows some of these barriers to break, and when they are broken, you get these beautiful ideas that give rise to challenging projects.”
Assistant Professor Suzanne Ponik from the UW Department of Cell and Regenerative Biology is exploring its potential as a treatment for breast cancer. “We’re interested in how PEG-FUD modifies the extracellular matrix of solid tumors and implications for drug delivery and immunotherapy,” Kwon explains.
The next stage of the project depends on funding. Kwon predicts that the team is still a few years off from being ready for first-in-human trials, but “we do think that PEG-FUD is manufacturable,” he notes.
While it’s been a long journey to make it this far, Tomasini-Johansson says it was worth it.
“I’m always struck by how important it is to do this basic science that maybe didn’t even have a clinical reason at the beginning, but that’s what you draw from to develop clinical tools,” she says. “It really shows the need to further public investment in basic research.”